Therapies that rescue patients with significant need
Eripio is Latin for rescue
Rescue is in our name
That is our purpose
Our technology has direct applicability in diseases with significant morbidity and mortality. Our goal is to use our platform to develop therapies that rescue patients from the threat of some of the most debilitating diseases known to man. These include:
- Heart disease and stroke
- Diabetes mellitus
- Fibrosis
- Alzheimer’s and Parkinson’s
- Autoimmunity
- Cancer
- Exposure to organophosphate pesticides and chemical nerve agents
We are an emerging biopharmaceutical company comprised of proven leaders in research, drug discovery and business operations with over 75 years of combined industry experience. We focus on the discovery of transformative therapies that, in collaboration with leading pharmaceutical companies, can efficiently deliver real solutions for the benefit of patients with unmet medical needs.
Our Vision
To discover and invent innovative biopharmaceuticals that empower patients to live their lives to the fullest.
Our Mission
To discover breakthrough therapeutics with clinically superior safety and efficacy attributes over existing treatments.
Our Goal
To be a leader in the discovery and early development of tomorrow’s biotherapeutics.
Our Focus
Theripion is focused on development of biologics that target important factors in lipid metabolism, oxidative stress and inflammation. Systemic delivery of our molecules is achievable with expected long half-life in blood. Laboratory development has shown predictable manufacturability. Preclinical results show promising effects in animal models.
Novel Biotherapeutics
|
Ther4
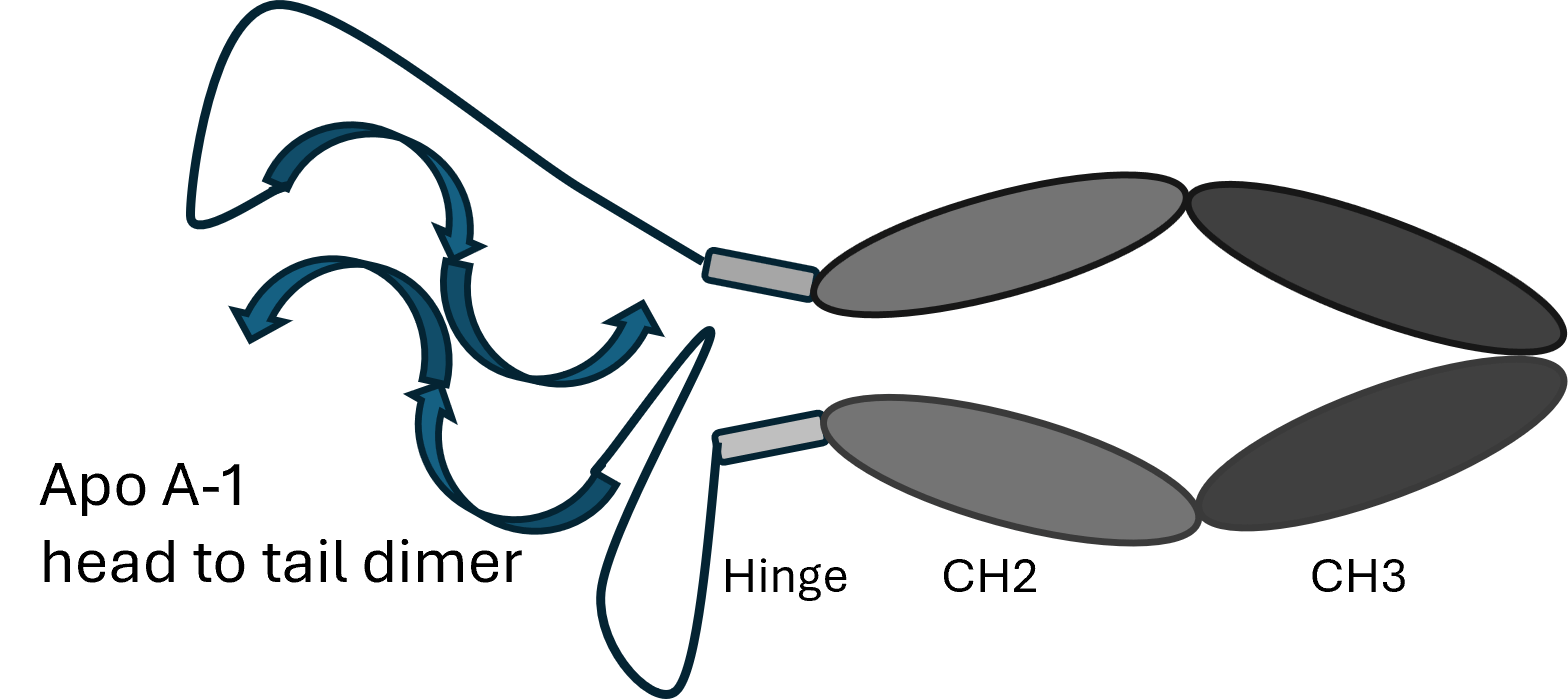
- Ther4 is an Apo A-I fusion protein providing the main protein associated with HDL
- Apo A-I typically has short half-life in the lipid-free state, whereas Ther4 has a longer half-life
- Even with significant lowering of LDL cholesterol, there is residual risk for coronary heart disease thought to be due to dysfunctional HDL
- Indication is to improve reverse cholesterol transport with potential to stabilize and/or reduce coronary plaques
|
TR-22
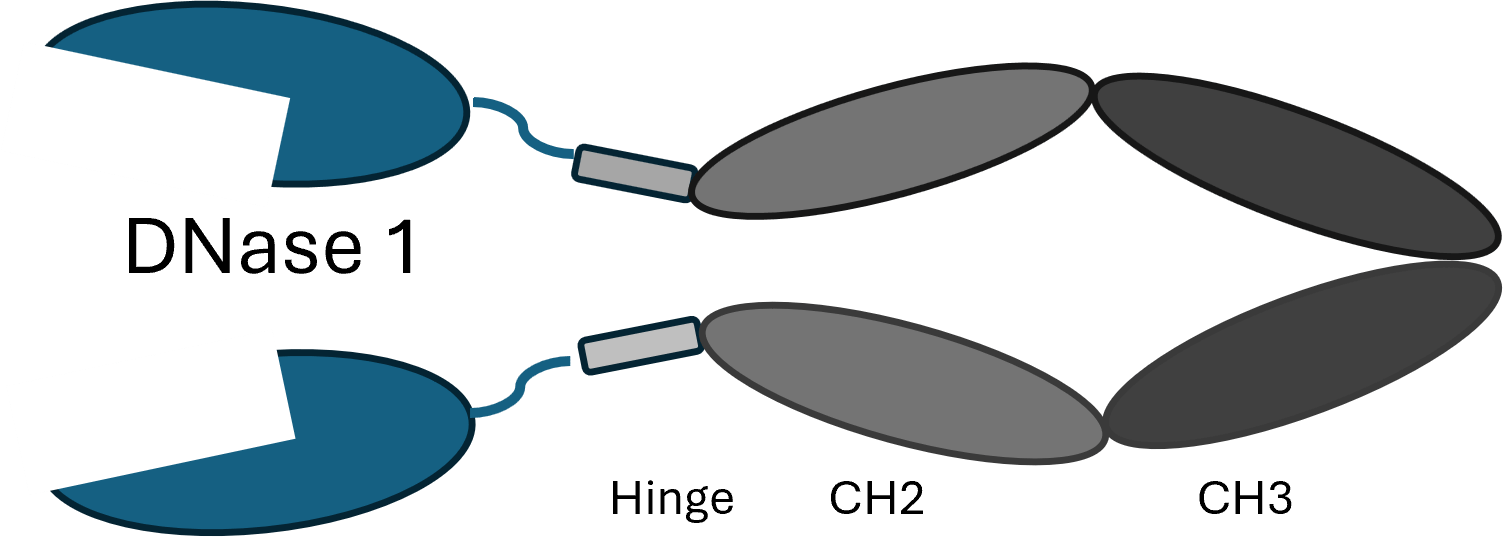
- DNase Fc molecule providing the anti-inflammatory enzyme deoxyribonuclease
- TR-22 is resistant to inhibition by actin and has a long half-life
- DNase exists as a treatment for cystic fibrosis delivered directly to the lungs via nebulization but is not effective when delivered systemically due to a short half-life
- Therapeutic implications exist for multiple inflammatory diseases including autoimmune conditions, cancer and even COVID-19
- A significant target of DNase is neutrophil extracellular traps (NETS) where many studies have shown that neutralization of NETS results in improvement in many diseases and improved efficacy when combined with current treatments
- Systemic delivery would be ideal to treat these conditions and the long half-life of our molecule provides this advantage
|
TR-43

- PON1 Fc molecule providing the antioxidant enzyme paraoxonase 1
- This is a first-in-class molecule providing a long half-life to this important enzyme
- The enzyme is deficient in many diseases including fibrosis and inflammation
- There is also potential of this enzyme to provide protection from harmful chemicals such as pesticides and chemical nerve agents
- There are promising data regarding prevention of diabetes in a rodent model of type 1 diabetes and treatment of insulin resistance in a different rodent model using recombinant PON 1
|
Paraoxonase Fusion Protein Platform

- Bispecific molecules combined with the antioxidant enzyme paraoxonase 1
- This is a first-in-class platform providing combination therapies for a multitude of diseases including heart disease and inflammatory conditions
- Apo A-I and PON1 are typically associated with HDL and provide many of its beneficial effects
Company Strategy
Discovery and Development
- Theripion, Inc. is a privately held, Seattle-based biotechnology company focused on targeted rescue enzyme therapy applicable to multiple diseases. We are at the pre-clinical stage optimizing our lead molecules with the goal of propelling our innovations into the clinic.
- We have focused on generation of intellectual property and have secured patent protection on Ther4 and bispecific Ther4-PON 1 fusion molecules in several jurisdictions with other patent applications pending.
- We have and continue to pursue preclinical studies in animal models of disease to provide proof-of-concept.
Commercialization
- We would like to collaborate with leading biopharmaceutical companies to continue clinical development of our lead molecules.
- We envision obtaining funding to pursue production of material for toxicity studies that can lead to an IND from the FDA.
- The following step would be to generate GMP material to take into the clinic for studies in man.
- We would like to partner with companies that can help propel our lead molecules into FDA approved products that provide significant help to patients in need of these therapies.
Company Leadership

Jeffrey Ledbetter, Ph.D.
CEO
Jeffrey A Ledbetter is an Immunologist who has contributed high impact research and inventions for the past 35 years. He has over 300 peer reviewed publications and his work has been cited over 50,000 times. He is an inventor of more than 100 patents, including patents on biological therapies for lymphoma and rheumatoid arthritis that are still used today.
Dr. Ledbetter was most recently a research professor of Rheumatology at the University of Washington where he helped invent RNase therapy for systemic lupus erythematosus, now in clinical trials. Dr. Ledbetter has also helped form several biotech companies including Genetic Systems (purchased by BMS), Trubion (purchased by Emergent), Resolve Therapies, and Theripion, Inc. where he now serves as CEO. Dr. Ledbetter earned his Ph.D. in cancer research from the University of Wisconsin, Madison, and did postdoctoral training at Stanford.

Martha Hayden-Ledbetter, Ph.D.
Chief Scientific Officer
Martha Hayden-Ledbetter is a molecular biologist and immunologist who earned her Ph.D. in Genetics at University of Washington. She has worked for large Pharma such as Johnson & Johnson and Bristol-Myers Squibb, and in academia at the University of Washington, Fred Hutchinson Cancer Research Center, and at Pacific Northwest Research Institute. She has also been involved in several Biotech startups, including Xcyte Therapies, Trubion Pharmaceuticals, and Theripion, Inc. Dr. Hayden-Ledbetter has contributed to multiple significant discoveries and inventions, including work on cytosine deaminase, scFvs, and HE4 in oncology. She also helped develop technology for construction of artificial receptors for T cell activation. Martha has developed and refined technologies for fusion protein design and expression in mammalian systems, working extensively on creating novel multi-functional molecules through innovative combinations of protein domains from heterologous molecules. One example is a nuclease fusion protein RNaseFc (RSLV132), licensed from the University of Washington to Resolve Therapeutics, that is being tested in the clinic for use in Sjogrens syndrome, SLE, and long COVID. Dr. Hayden-Ledbetter is currently working on fusion proteins of apolipoproteins and on enzyme fusion proteins, including the enzymes paraoxonase and DNase1. She has filed multiple patent applications, has more than 30 issued patents, and 25 peer reviewed publications.

Vince Montes, MD
Chief Medical Officer
Senior VP for Research and Development
Vince Montes is a physician-scientist with board certification in Endocrinology. He completed his fellowship in Endocrinology at the University of Washington in Seattle in 2012. He has been practicing medicine since graduating from medical school in 2001. He has clinical expertise in the settings of academics, hospital medicine and private practice. He is an inventor on Theripion patents. He has a research background in metabolic disease and inflammation, having published research, clinical reports and a book chapter in these areas. He continues clinical practice in conjunction with company research duties. His ability to combine clinical knowledge with basic science research puts him in a unique position to contribute to the translational aspects of the company’s goals.

Randall B. Riggs, MBA
Business Development Advisor
Randall B. Riggs has over twenty-seven (27) years of corporate strategy and business development experience within the pharmaceutical and biotechnology industries. From 2016 – present he actively provides strategic business development consulting services for several innovative biotechnology companies. From 2007 – 2016, he was the President & CEO of Advanced Cancer Therapeutics where he created the infrastructure, in-licensed several novel anti-cancer products and raised $15 million. From 2005 to October of 2007 he was the Senior Vice President of Corporate Development for BioCryst Pharmaceuticals and was responsible for approximately $1 billion dollars in strategic partnerships with multi-national firms including Hoffman-La Roche, Shionogi & Co., Mundipharma International LTD, and Green Cross Corp. Prior to BioCryst Pharmaceuticals, Mr. Riggs held the position of Senior Vice President of Corporate Development for Lexicon Pharmaceuticals (1998 – 2004). As a member of the Executive Management Team, over $200 million was raised through their initial public offering in April 2000. In addition to his key role in Lexicon’s successful IPO, Mr. Riggs oversaw an increase in strategic partnership revenue from less than $2 million to over $60 million each year. Mr. Riggs began his career in the pharmaceutical industry at Eli Lilly & Company in 1992 in sales and sales management, and was quickly promoted to Manager in Corporate Business Development at corporate headquarters in Indianapolis, Indiana. During his career at Eli Lilly, he played a leading role in the evaluation of several strategic acquisitions, product in-licensing opportunities, and establishment of several collaborations with biotechnology companies. Mr. Riggs received his Bachelor of Business Administration (BBA) in Management cum laude from Texas A&M University and his Masters in Business Administration (MBA) in Finance from the University of Houston.
Scientific Advisors

Alan Chait, MD
Professor and Former Chair, Division of Metabolism, Endocrinology and Nutrition, University of Washington, Seattle, WA

Keith Elkon, MD
Professor and Former Chair, Division of Rheumatology, University of Washington, Seattle, WA
News & Updates
Theripion, Inc. granted patents for human apolipoprotein A-I fusion protein technology
Ongoing
Patents have been granted in Europe, Israel, Australia, New Zealand, and Japan. Applications have been filed in the US, Canada, China and South Korea and remain pending.
DNase fusion protein shows positive results in mouse model of breast cancer
December 2021
DNase fusion protein from Theripion, Inc., shows reduced tumor volume in a mouse model of breast cancer when used alone or added to currently used immunotherapy.
Apo A-I and paraoxonase fusion proteins show positive results in mouse model of pulmonary fibrosis.
January 2021
Fusion proteins from Theripion, Inc., show improvements in bleomycin induced pulmonary fibrosis in mice.
